1. Introduction
The delectable and succulent papaya fruit, a member of the Caricaceae family, is scientifically recognized as
Carica papaya Linn. This fruit thrives in diverse regions across the globe, including the tropical climates of America and Europe, as well as in the fertile lands of India
| [1] | Anuar, N. S., et al., Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food and Chemical Toxicology, 2008. 46(7): p. 2384-2389. https://doi.org/10.1016/j.fct.2008.03.025 |
[1]
. Cultivated extensively in both tropical and subtropical regions, papaya stands as one of the most significant fruit crops. This fruit is abundant in three potent antioxidant vitamins: C, A, and E, as well as minerals like magnesium and potassium. Furthermore, it is a rich source of vitamin B, pantothenic acid, folate, and fiber. Notably, papaya contains the digestive enzyme papain, which has proven effective in treating various ailments, including trauma, allergies, and sports injuries. Collectively, these essential nutrients contribute to improved cardiovascular health, offering protection against heart diseases, heart attacks, strokes, and colon cancer
| [2] | Aravind, G., et al., Traditional and medicinal uses of Carica papaya. Journal of medicinal plants studies, 2013. 1(1): p. 7-15. |
[2]
.
Papain (EC 3.4.22.2), a plant cysteine protease enzyme, is derived from the latex of papaya (
Carica papaya L.) through a process of isolation. This enzyme is extracted by slicing the skin of unripe papaya fruit and subsequently collecting and drying the exuded latex. Notably, the greener the fruit, the higher the papain activity. As a member of the papain superfamily, this proteolytic enzyme plays a pivotal role in various essential biological processes across all living organisms
| [3] | Tsuge, H., et al., Inhibition mechanism of cathepsin L-specific inhibitors based on the crystal structure of papain–CLIK148 complex. Biochemical and Biophysical Research Communications, 1999. 266(2): p. 411-416. https://doi.org/10.1006/bbrc.1999.1830 |
[3]
. Papain exhibits significant proteolytic activity on a range of substrates, including proteins, short-chain peptides, amino acid esters, and amide bonds. Owing to its versatile catalytic properties, papain has found widespread applications in the food and medical sectors. Notably, this enzyme demonstrates a preferential cleavage of peptide bonds involving basic amino acids, with a particular affinity for arginine, lysine, and residues succeeding phenylalanine
| [4] | Menard, R., et al., A protein engineering study of the role of aspartate 158 in the catalytic mechanism of papain. Biochemistry, 1990. 29(28): p. 6706-6713. https://doi.org/10.1021/bi00480a021 |
[4]
. The distinct structural characteristics of papain are intrinsically linked to its functional attributes, providing valuable insights into the proteolytic mechanisms of this enzyme. Understanding these mechanisms is key to unlocking papain's diverse range of applications
| [5] | John Sundar, V., et al., Recovery and utilization of proteinous wastes of leather making: a review. Reviews in Environmental Science and Bio/Technology, 2011. 10: p. 151-163. https://doi.org/10.1007/s11157-010-9223-6 |
[5]
.
Ethiopian leather industry is recognized as a significant contributor to environmental pollution, primarily due to the substantial amounts of liquid and solid waste it generates. Tanning, the process of transforming raw hides and skins into imputrescible materials, is particularly noteworthy in this context. Operations such as reduction, leveling, and purification produce both untanned and tanned proteinaceous waste, which, if not effectively managed, can lead to significant environmental challenges. The degradation of proteinaceous molecules in these solid wastes results in the emission of unpleasant odors and greenhouse gases, including NH3, H2S, CH4, and CO2, further exacerbating the negative environmental impacts of this industry
| [6] | Urgessa, O. E., D. D. Itana, and T. O. Raga, Extraction of papain from papaya (Carica papaya L.) fruit latex and its application in transforming tannery raw trimming. Ethiopian Journal of Science and Sustainable Development, 2019. 6(2): p. 22-32. https://doi.org/10.20372/ejssdastu:v6.i2.2019.92 |
[6]
.
Plant latex-derived proteolytic enzymes have garnered increased interest due to their broad substrate specificity and ability to maintain activity across a wide range of pH levels and temperatures. Furthermore, these enzymes exhibit remarkable resilience in the presence of organic compounds and various additives, making them particularly valuable for diverse applications
| [7] | Tomar, R., R. Kumar, and M. Jagannadham, A stable serine protease, wrightin, from the latex of the plant Wrightia tinctoria (Roxb.) R. Br.: purification and biochemical properties. Journal of agricultural and food chemistry, 2008. 56(4): p. 1479-1487. https://doi.org/10.1021/jf0726536 |
[7]
. Isolating and purifying papain in its native crystalline state from fresh latex is crucial for industrial applications. To achieve this, several well-established methods have been developed. These methods typically involve a combination of techniques, such as ATPS (Aqueous Two-Phase System) and Sephadex G-75-based approaches, followed by various drying procedures. By employing these isolation and purification methods, papain can be effectively extracted and purified from green papaya fruits. This ensures that the enzyme maintains its stability and functionality, making it suitable for a wide range of applications in the food, pharmaceutical, and leather industries, among others
| [8] | Paul, B., et al., Isolation, purification and modification of papain enzyme to ascertain industrially valuable nature. International Journal of Bio-Technology and Research, 2013. 3(5): p. 11-22. |
[8]
.
In an effort to reduce environmental impact, the leather industry has adopted cleaner production methods aimed at minimizing chemical waste, water consumption, and raw material loss. Global leather processing generates around 15 million tons of hides and skins annually, with an average daily wastewater discharge exceeding 15,000 million liters and an estimated 6 million tons of solid waste produced yearly
| [9] | Hashem, M. A., M. N. Z. Khan, and P. Roy, oxidizers effect of sulphide removal from hair dissolving liming wastewater in tannery. risk. 2: p. 2. |
[9]
. Proper disposal of 4.5 million tons of sludge and treatment plant effluents poses a significant challenge. Moreover, Lime/sulfide remains a popular hair removal method due to its efficiency and cost-effectiveness compared to alternative technologies. Chromium salts are commonly employed as tanning agents. Sulfur compounds found in effluents originate from organic matter, primarily hair, as well as from processing chemicals such as surfactants, unhairing agents like sodium sulfide (Na2S), sulfates, and sulfides. Hydrogen sulfide (H2S) poses a risk due to its toxic and corrosive properties, necessitating appropriate management and treatment strategies
| [10] | Souza, F. and G. Mariliz, Application of enzymes in leather processing: A comparison between chemical and coenzymatic processes. Brazilian Journal of Chemical Engineering, 2012. 29: p. 473-482. https://doi.org/10.1590/S0104-66322012000300004 |
| [11] | Sivaram, N. M. and D. Barik, Chapter 5 - Toxic Waste From Leather Industries, in Energy from Toxic Organic Waste for Heat and Power Generation, D. Barik, Editor. 2019, Woodhead Publishing. p. 55-67. https://doi.org/10.1016/B978-0-08-102528-4.00005-5 |
[10, 11]
. As a response to these challenges, researchers have turned to bio-based dehairing agents, such as enzymes derived from
Carica Papaya. Moreover, despite promising results, the practical application of Carica Papaya enzymes in the leather industry is hindered by the lack of optimized enzyme extraction and purification methods, leading to low dehairing efficiency.
The 21
st century has witnessed a significant expansion of biotechnology into various commercially valuable and complex biochemical processes. Enzyme technology, a critical branch of biotechnology, has emerged as a key player in streamlining numerous industrial procedures. By harnessing the power of enzymes, industries can improve process efficiency, reduce costs, and simplify operations, leading to substantial benefits and advancements across multiple sectors
| [12] | Tigist, M., et al., Extraction and purification of papain enzyme from papaya leaf and the phytochemical components of the leaf. Biotechnology International, 2016. 9(8): p. 176-104. |
[12]
. In this regard, enzymes offer themselves as attractive alternatives for greening leather processing
| [13] | Alexander, K., Enzymes in the tannery: catalysts for progress? Remote sensing of environment (USA), 1988. |
[13]
. Use of proteases for bating process in leather production is a well-established commercial practice, and in fact, has been in vogue for more than a century
| [14] | Deselnicu, M., et al., A new enzyme process for improved yield and softer leather. The Journal of the American Leather Chemists Association (USA), 1994. |
| [15] | Jaouadi, N. Z., et al., A novel keratinase from Bacillus tequilensis strain Q7 with promising potential for the leather bating process. International journal of biological macromolecules, 2015. 79: p. 952-964. https://doi.org/10.1016/j.ijbiomac.2015.05.038 |
[14, 15]
. Only recently, enzyme technology is being seriously considered for replacing polluting chemicals in other stages of leather processing. The last two decades have witnessed increasing use of enzymes in soaking
| [16] | Pfleiderer, E., Auxiliary agents for the bovine tannery beamhouse. Leather, 1985. 187(2): p. 14-18. |
[16]
and degreasing
| [17] | Palop, R., A. Marsal, and J. Cot, Optimization of the aqueous degreasing process with enzymes and its influence on reducing the contaminant load. Journal of the society of leather technologists and chemists, 2000. 84(4): p. 170-6. |
[17]
. Application of proteases for dehairing
| [18] | Hammami, A., et al., Proteolytic and amylolytic enzymes from a newly isolated Bacillus mojavensis SA: characterization and applications as laundry detergent additive and in leather processing. International journal of biological macromolecules, 2018. 108: p. 56-68. https://doi.org/10.1016/j.ijbiomac.2017.11.148 |
[18]
.
In recent decades, technological advancements and increased awareness of plant-based enzymes have led to a growing demand for enzyme extraction and isolation. Consequently, there is a pressing need to increase the catalytic efficiency of enzymes derived from various plant parts to meet industrial requirements. In this context, the present study focuses on optimizing the extraction and isolation of enzymes from Carica Papaya using a response surface methodology (RSM) approach. The primary objective is to enhance the efficiency of dehairing processes in the leather industry. In this sector, enzymes play a crucial role in minimizing environmental pollution compared to conventional chemical-based methods. By combining advanced RSM techniques with traditional pharmacognostic methods, this research aims to contribute to the development of sustainable and effective enzyme-based solutions for the leather processing sector. Furthermore, the findings of this study could have broader implications for the extraction and isolation of enzymes from other plant sources, further bolstering the potential of plant-derived enzymes in various industries.
2. Materials and Method
2.1. Study Area, Period, and Design
A laboratory-based experimental study was conducted at Arba Minch University's Microbiology and Parasitology Laboratory from April to August 2023. Carica Papaya fruits were collected from various locations within Arba Minch town and its surrounding district for the preparation of crude proteolytic enzymes. Arba Minch is situated in the Southern Nations, Nationalities, and Peoples' Region (SNNPR) of Ethiopia and serves as the administrative center for the Gamo Zone. The town is located at 30°56'N of the equator and 37°44'E, approximately 505 km southwest of Addis Ababa. Arba Minch covers a surface area of 2184 hectares, with altitudes ranging from 1200 to 1400 meters above sea level. The town experiences an average temperature of approximately 25°C and receives an annual rainfall of around 575 mm, making it an ideal location for the cultivation and collection of Carica Papaya fruits for enzyme production
| [19] | Dejene, F., B. Regasa Dadi, and D. Tadesse, In vitro antagonistic effect of lactic acid bacteria isolated from fermented beverage and finfish on pathogenic and foodborne pathogenic microorganism in Ethiopia. International journal of microbiology, 2021. 2021(1): p. 5370556. https://doi.org/10.1155/2021/5370556 |
[19]
.
Figure 1. Location Map of Arba Minch town and its district.
2.2. Laboratory Procedure
2.2.1. Sample Collection
The samples were purposively and aseptically collected by using sterilized ice box containers from different areas of Arba Minch town and district. Afterward, the fruit of the plant was stored at 4°C until the extract was prepared.
2.2.2. Preparing the Proteolytic Enzyme Extract C. Papaya
According to Phanuphong Chaiwut and his collegue
| [20] | Chaiwut, P., et al., A comparative study on properties and proteolytic components of papaya peel and latex proteases. Chiang Mai J. Sci, 2007. 34(1): p. 109-118. |
[20]
Papaya peels was prepared by peeling the fruits and cutting them into small pieces. The fresh papaya was grounded in a blender before mixed with the extraction media (H
2O and 50 mM phosphate buffers, pH 7.0) at a ratio of 1:9 (w/v), and it was then let be stand for 30 min. The extracted samples were then centrifuged at 9000 × g at 4
◦C for 20 min. The obtained supernatant was filtered through a Whatman paper No. 1. This sample was be referred to as the crude extract and used for further experiment.
2.2.3. Caseinolytic Activity Assay
To assess enzyme activity, 0.1 mL samples were combined with 1% w/v casein in 1.1 mL reaction buffer and incubated. At specific time points, up to 2 minutes, the reaction was halted by introducing 5% w/v trichloroacetic acid (TCA) (1.8 mL). Following centrifugation, the supernatant's absorbance was measured at 280 nm. Blanks were prepared by first adding TCA to the enzyme, followed by the substrate. The caseinolytic unit (UC) was determined using a previously established method
| [21] | Lopéz, L. M., et al., Latex peptidases of Calotropis procera for dehairing of leather as an alternative to environmentally toxic sodium sulfide treatment. Bioprocess and biosystems engineering, 2017. 40: p. 1391-1398. https://doi.org/10.1007/s00449-017-1796-9 |
[21]
.
Enzyme Activity =
µg / mL of tyrosine equivalents released×dilution factor Volume of enzyme used (mL)×Time of assay (minutes) Enzyme Activity =
µg / mL of tyrosine equivalents released×dilution factor Volume of enzyme used (mL)×Time of assay (minutes) 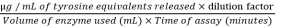 (1)
(1) The enzyme activity is expressed in Units / mL enzyme
µg / mL of tyrosine equivalents released = actual OD/slope of standard graph
Actual OD = Reaction Mixture OD - Reaction blvvank OD
2.2.4. Optimization of Enzyme Activity
According to
| [22] | Faber, E. J., et al., Characterization of the exopolysaccharide produced by Streptococcus thermophilus 8S containing an open chain nononic acid. European journal of biochemistry, 2002. 269(22): p. 5590-5598. https://doi.org/10.1046/j.1432-1033.2002.03266.x |
| [23] | Sharma, K., et al., Optimization of various process parameters using response surface methodology for exopolysaccharide production from a novel strain Pediococcus acidilactici KM0 (Accession Number KX671557) isolated from milk cream. International Journal and Emerging Research Management and Technology, 2017. 6: p. 2278-9359. |
[22, 23]
, The Response Surface Methodology (RSM) is an empirical statistical technique employed to elucidate the individual and combined effects of various factors, such as pH, reaction time, and temperature, during the optimization process. This methodology facilitates multiple regression analysis by simultaneously solving multivariate equations. The quantitative data derived from designed experiments serves as the basis for these calculations. RSM is particularly valuable for optimizing processes involving multiple variables, as it allows for the exploration of their interactive effects and the identification of optimal conditions.
In order to examine the impact of factors such as temperature (A), pH (B), and reaction time (C) on enzyme activity, a quadratic model equation was developed using rough approximations. The experimental design process employed Design Expert software (Stat Ease, 11 trial editions), which facilitated the generation of the quadratic model. Response Surface Methodology (RSM) was employed due to its significant benefits, such as its ability to efficiently analyze various parameters and their interactions using fewer experimental trials. RSM also plays a critical role in optimizing, enhancing, and refining processes. The Box Behnken Design (BBD) is one of the most frequently used experimental designs within RSM. In this study, the BBD model was applied to investigate two process parameters. Three independent variables were considered: pH (ranging from 2 to 13), temperature (ranging from 15°C to 95°C), and contact time (ranging from 7 to 70 minutes). The enzyme activity served as the response variable in this investigation. The utilization of BBD within the RSM framework allowed for a comprehensive analysis of the combined effects of these variables on enzyme activity.
The quadratic equation model's optimal point is expressed by Eq. (
2), as derived from the Response Surface Methodology (RSM) analysis.
where Y is the predicted response, xi, xj,..., xk are the input variables which affect the response Y, x2 i, x2 j,..., x2 k are the square effects, xixj, xixk and xjxk are the interaction effects, b0 is the intercept term, bi (i = 1, 2,..., k) is the linear effect, bii (i = 1, 2,..., k) is the squared effect, bij (i = 1, 2,..., k; j = 1, 2,..., k) is the interaction effect and e is the statistical error.
The chosen independent variables used in this study were coded according to
| [24] | Banik, R., A. Santhiagu, and S. Upadhyay, Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresource technology, 2007. 98(4): p. 792-797. https://doi.org/10.1016/j.biortech.2006.03.012 |
[24]
Eq
3:
X
Coded=
X
Coded=
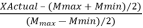 (3)
(3) Where x Coded is the dimensionless coded value of the independent variable, XActual is the actual value of the independent variable, and Mmax and Mmin are the High and Low independent variables.
Table 1. Process variables considered in the RSM design and their levels.
Factor | Symbol | Level |
Coded | Uncoded | Coded | Uncoded |
Temperature | X1 | A | 1 | 15 |
| | | 0 | 55 |
| | | -1 | 95 |
pH | X2 | B | 1 | 13 |
| | | 0 | 7.5 |
| | | -1 | 2 |
Reaction Time | X3 | C | 1 | 70 |
| | | 0 | 38.5 |
| | | -1 | 7 |
2.2.5. Dehairing Assay at the Laboratory Scale
Fresh raw goat skins were obtained from local slaughterhouses in Arba Minch town. The skins were thoroughly washed with sodium chloride and water to remove any impurities and then dried in a hot air oven at 50°C. To prepare the hide for further processing, the salted hide was cut into 4 g pieces (wet weight), each measuring approximately 9 cm². These hide pieces were soaked in 40 mL crude enzyme solutions in a petri dish, followed by incubation at 25°C for 24 hours. A control assay was performed under identical conditions, except for the absence of the proteolytic preparation. After the incubation period, the hide pieces were carefully removed and cleaned with water. The hairs were eliminated using a standardized gentle scraping method, allowing for a detailed examination of the dehairing results
| [25] | Errasti, M. E., et al., Plant proteases from Carica papaya and Vasconcellea quercifolia with potential application for a cleaner processing in tanneries. Biocatalysis and Biotransformation, 2020. 38(5): p. 357-366. https://doi.org/10.1080/10242422.2020.1751131 |
[25]
.
2.2.6. Microscopy Analysis
Following a 24-hour incubation period, the hide pieces were carefully removed and cleaned with water. The hairs were then eliminated using a standardized gentle scraping technique. To ensure thorough hair removal, the process was examined under a magnifying glass. Next, 1 cm² samples of the incubated hide were obtained by cutting, after which they were washed and fixed in formal saline solution for further analysis
| [25] | Errasti, M. E., et al., Plant proteases from Carica papaya and Vasconcellea quercifolia with potential application for a cleaner processing in tanneries. Biocatalysis and Biotransformation, 2020. 38(5): p. 357-366. https://doi.org/10.1080/10242422.2020.1751131 |
[25]
.
2.2.7. Physico-Chemical Analysis After the Enzymatic Process
Effluents generated from both conventional and enzymatic processes were collected for further analysis. The physico-chemical properties of these effluents, including biochemical oxygen demand (BOD) and chemical oxygen demand (COD), were examined to assess the environmental impact of each process
| [26] | Ben Elhoul, M., et al., Heterologous expression and purification of keratinase from Actinomadura viridilutea DZ50: feather biodegradation and animal hide dehairing bioprocesses. Environmental Science and Pollution Research, 2021. 28: p. 9921-9934. https://doi.org/10.1007/s11356-020-11371-1 |
[26]
.
2.2.8. Physical Testing
To evaluate the morphological properties of enzymatically and conventionally processed leathers, visual assessment and standard procedures were employed, with a focus on quality and strength attributes. The leather samples were first humidified to 65% for 48 hours at 26 ± 2 °C. Following this, standard methods were used to determine various physical parameters of the crust leather, including tear force, tensile power, and lengthening percentage
| [26] | Ben Elhoul, M., et al., Heterologous expression and purification of keratinase from Actinomadura viridilutea DZ50: feather biodegradation and animal hide dehairing bioprocesses. Environmental Science and Pollution Research, 2021. 28: p. 9921-9934. https://doi.org/10.1007/s11356-020-11371-1 |
[26]
.
2.3. Data Analysis
The study involved conducting all experiments in triplicates to ensure reliability and accuracy. Following the experiments, the collected data was thoroughly verified, cleaned, and coded to guarantee its completeness. Microsoft Excel 2010 was utilized for data entry, after which the data was exported to the Statistical Package for Social Sciences (SPSS) version 25 for further analysis. Descriptive statistics, such as the mean, were employed to illustrate enzyme activity. Data analysis was performed using Design-Expert version 11, incorporating one-way ANOVA to identify significant differences between groups. A P-value of ≤ 0.05 was established as the threshold for determining statistical significance. This comprehensive approach ensured accurate data analysis and interpretation, leading to valid conclusions regarding enzyme activity.
Figure 2. Infographics representation of this study.
3. Result and Discussion
3.1. The Enzyme Activity Assay for Crude Extract of Papain
The proteolytic enzyme activity of
Carica papaya crude extract was examined utilizing casein as a substrate.
Table 2 demonstrates that the papain-derived crude enzyme exhibited an enzyme activity of 10.5 U/ml. additionally; the protein concentration of the
Carica papaya crude enzyme was determined to be 2.5 mg/ml.
Table 2. Protein concentrations determined by the Lowry Method, along with their enzyme activities.
Crude Enzyme | Protein concentration (mg/ml) | Enzyme activity (U/ml) |
Papain | 2.5 | 10.5 |
A unique proteolytic enzyme known as papain is produced by the Carica papaya plant. According to standard protocols, the papain-rich juice was extracted from the papaya fruits through a process involving crushing the fruits in a grinder, pressing the resulting mass, and separating the juice. Crude enzyme extraction was performed utilizing the fruit juice obtained from papaya plants. The efficacy of these crude extracts was assessed using keratin digestion units (U/ml), which provide an estimate of their keratin proteolytic activity.
When we go right into our finding, 10.5 U/ml enzyme activities are observed in a carica papaya crude enzyme juice extract. Comparable research from Argentina shows that papain has proteolytic activity on the keratin substrate with the enzyme activities of 5 U/ml
| [25] | Errasti, M. E., et al., Plant proteases from Carica papaya and Vasconcellea quercifolia with potential application for a cleaner processing in tanneries. Biocatalysis and Biotransformation, 2020. 38(5): p. 357-366. https://doi.org/10.1080/10242422.2020.1751131 |
[25]
. On the other hand, the protein concentration (papain) of crude extract was determined by Lowry’s method. A protein concentration of papain was found to be 2.5 mg/ml for crude extract. Research done in India depict that the Protein concentration of papain fruit juice was determined as 1.11 mg/ml, 0.39 mg/ml and 0.14 mg/ml for crude extract
| [27] | Gautam, S., et al., Comparative study of extraction, purification and estimation of bromelain from stem and fruit of pineapple plant. The Thai Journal of Pharmaceutical Sciences, 2010. 34(2): p. 67-76. |
[27]
. As a result, we can infer from the study's results that
C. papaya is a good source of plant proteases with potential for hair removal as well as for a cleaner tanning process.
3.2. Dehairing Experiment
A study was carried out to examine the potential advantages of utilizing
Carica papaya crude enzyme over chemical methods for dehairing leather, as chemicals can compromise leather quality and cause damage. Separate leather pieces were immersed in enzyme and chemical solutions (Na
2S) for a duration of 16 hours.
Figure 3 illustrates that 100% dehairing was achieved with enzymes, yielding superior results compared to chemicals after 12 hours, without causing any noticeable damage to the leather.
Figure 3. Dehairing assay (A: chemical dehairing of goat skin; B&C: enzymatic dehairing of goat skin; D: collagen in the grain layer recorded from chemically dehaired goat skin; E: undamaged goat hair from goat skin; the images were captured with a stereo microscope, LEICA M205 C).
The results demonstrated that Na2S caused damage to the leather after 16 hours of exposure. Furthermore, a rough texture was observed when chemical treatment was applied to the hair pulp. The findings of the study revealed that the crude enzyme derived from papaya exhibited superior dehairing performance on hides at the 12-hour mark compared to chemical treatment.
Figure 3(E) highlights the remarkable efficiency of papaya crude enzyme in removing hair from goat skin, while preserving the morphological structure of the collagen layer, making it an ideal choice for the dehairing process. In contrast, the chemical treatment with Na2S was found to negatively impact the collagen in the grain layer to some extent, potentially leading to undesirable properties in the finished leather, as illustrated in
Figure 3(D).
The dehairing ability of
C. papaya protease was evaluated using goat hide as a substrate. The initial dehairing process was carried out for 16 hours with a constant enzyme concentration. The results showed that enzyme dehairing effectively removed the epidermis along with the hair, whereas chemical dehairing only partially removed the epidermis, leaving short hairs visible in certain areas. Enzyme-dehaired skins exhibited a smoother, cleaner, and whiter appearance due to the complete removal of the epidermis. Moreover, the collected hair remained intact during the enzyme dehairing process. Similar findings from earlier experiments carried out in Argentina revealed that the dehairing activity of papain made the skin smoother and cleaner after being enzymatically dehaired
| [25] | Errasti, M. E., et al., Plant proteases from Carica papaya and Vasconcellea quercifolia with potential application for a cleaner processing in tanneries. Biocatalysis and Biotransformation, 2020. 38(5): p. 357-366. https://doi.org/10.1080/10242422.2020.1751131 |
[25]
. Similar to this, Roy and his colleague also show how the papain enzyme dehairs cow skin
. Therefore, it was shown that papain is comparable to keratinase enzyme in terms of dehairing process utilized in the leather industry.
3.3. RSM Approach for Optimizing Papain Enzyme Activity
To identify the optimal conditions for the three key variables (temperature, pH, and reaction time), a Box-Behnken design (BBD) was employed with proteolytic enzyme activity as the output response. The study comprised 17 experiments, which explored various combinations of the selected variables.
Table 3 provides a detailed overview of the experimental designs utilized in this investigation. By employing this systematic approach, the study aimed to elucidate the most favorable conditions for maximizing the proteolytic enzyme activity.
Table 3. Optimization of proteolytic enzyme activity by C. papaya crude enzyme using Box Behnken design (BHD).
Run | Temperature (oC) | pH | Time (min) | Response Activity (U/ml) | Residual |
Predicted | Actual |
1 | 1 | 1 | 0 | 22.25 | 22.25 | 0.0000 |
2 | -1 | 1 | 0 | 26.75 | 26.75 | 0.0000 |
3 | -1 | 0 | 1 | 20.75 | 20.75 | 0.0000 |
4 | 0 | -1 | 1 | 21.50 | 21.5 | 0.0000 |
5 | 1 | 0 | 1 | 21.25 | 21.25 | 0.0000 |
6 | 0 | 1 | -1 | 25.50 | 25.5 | 0.0000 |
7 | 0 | 0 | 0 | 30.80 | 31 | 0.2000 |
8 | -1 | -1 | 0 | 20.75 | 20.75 | 0.0000 |
9 | 1 | -1 | 0 | 16.25 | 16.25 | 0.0000 |
10 | 0 | 0 | 0 | 30.80 | 31 | 0.2000 |
11 | 0 | 0 | 0 | 30.80 | 32 | 1.20 |
12 | 1 | 0 | -1 | 14.25 | 14.25 | 0.0000 |
13 | 0 | 1 | 1 | 25.50 | 25.5 | 0.0000 |
14 | 0 | 0 | 0 | 30.80 | 30 | -0.8000 |
15 | 0 | -1 | -1 | 17.50 | 17.5 | 0.0000 |
16 | -1 | 0 | -1 | 23.75 | 23.75 | 0.0000 |
17 | 0 | 0 | 0 | 30.80 | 30 | -0.8000 |
The Response Surface Methodology (RSM) analysis of variance (ANOVA) revealed that papaya enzyme activity was influenced by three parameters, as outlined in
Table 4. The model's R-squared value (R
2 = 0.9942) and adjusted R-squared value (adj R
2 = 0.9867) were both found to be extremely close to 1, signifying a strong correlation between observed and predicted values. The predicted R-squared (pred. R
2) of 0.9909 was also deemed acceptable in comparison with R
2 and adjusted R
2. A signal-to-noise ratio greater than four is considered desirable for adequate precision, and this study yielded a high signal-to-noise ratio of 34.119. These results indicate that the experiments demonstrated sufficient precision and reliability, making the model suitable for navigating the design space.
Table 4. Model Performance, Precision, and Variability in ANOVA Analysis for Enzyme Activity by Crude Papaya.
Model developed for design space | R2 | (adj. R2) a | (pred. R2) b | Adequate Precision | SDc | CVd |
Enzyme activity by crude papaya | 0.9942 | 0.9867 | 0.9909 | 34.1187 | 0.6325 | 2.62 |
a: adjusted R-squared value
b: predicted R-squared value
c: SD: standard deviation value
d: CV: coefficient of variation value
3.4. Model Developed for Enzyme Activity in Terms of Coded Factors
Three independent variables were used to study the responses showing observed and predicted proteolytic enzyme activity. Based on the multiple regression analysis of the observed responses, the following quadratic equation was obtained:
Y= 30.80+3A-2.25B+1C+0.0000AB-1AC+2.5BC-3.4A2-5.9B2-4.9C2(4)
Where, Y= Response activity for Enzyme activity
The relationship between the response and the three significant variables, temperature (A), pH (B), and reaction time (C), can be represented using the following quadratic equation with coded factors. In this equation, high factor levels are denoted as +1, while low factor levels are denoted as -1. By examining the factor coefficients, one can ascertain the relative influence of each factor on the response. The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients.
Table 5 depicts the statistical significance of the quadratic model, considering F-values and probability values (prob. > F). Model terms with prob. > F less than 0.05 are deemed statistically significant. The developed model for enzyme activity revealed significant main effects (A, B, C) and quadratic effects (A
2, B
2, and C
2). Furthermore, the AC and BC interactions emerged as significant model terms, while the AB interaction was not significant. The model's F-value of 132.91 supports the significance of these terms. Additionally, the lack of fit's insignificance (prob. > F > 0.05) implies that the quadratic model is suitable for describing enzyme activity in the context of crude papaya.
Table 5. Significance of factors and interactions in ANOVA analysis for Enzyme Activity by Crude Papaya.
Model developed for design space | A | B | C | AB | AC | BC | A2 | B2 | C2 | Model |
Enzyme activity | < 0.0001a | < 0.0001a | 0.0029a | 1.0000b | 0.0159a | < 0.0001a | < 0.0001a | < 0.0001a | < 0.0001a | < 0.0001a |
a: Significant model terms are denoted, with C.V. representing the coefficient of variance and DF representing the degree of freedom.
b: Non-significant model terms are indicated.
The statistical significance of the quadratic model is depicted in
Table 5, which considers F-values and probability values (prob. > F). Model terms are deemed statistically significant if their prob. > F is less than 0.05. The enzyme activity model revealed that the main effects (A, B, C) and quadratic effects (A2, B2, and C2) were significant. Additionally, the AC and BC interactions emerged as significant model terms, while the AB interaction was not significant. The model's F-value of 132.91 supports the significance of these terms. The insignificance of the lack of fit (prob. > F > 0.05) suggests that the quadratic model is suitable for describing enzyme activity in crude papaya.
3.5. Predicting the Optimum Variable for the Maximum Enzyme Activity
As demonstrated in
Table 6, the highest enzyme activity was recorded in run 11, which corresponds to the following conditions: pH 7.5, temperature 55°C, and time 38.5 minutes. These findings indicate that this specific combination of variables defines the optimal region for achieving the highest enzyme activity levels. Therefore, controlling the experimental parameters within this range is essential for maximizing enzyme activity and overall efficiency.
Table 6. Optimum enzyme activity of papain enzyme against the substrate the casein.
Response | Predicted Mean | Predicted Median | Std Dev | SE Pred | Optimum pH | Temperature (oC) | Reaction time |
Enzyme activity | 30.8 | 30.8 | 0.632456 | 0.69282 | 7.5 | 55 | 38.5 |
Two-sided Confidence = 95%
3.6. Effect of Variables on Enzyme Activity
3.6.1. Effects of Temperature and pH
The interaction between temperature and pH is effectively visualized through two-dimensional contour plots and three-dimensional (3D) plots (
Figure 4A and B). As evident in the contours and 3D plots, a rise in enzyme activity was observed with increasing concentration and pH. By superimposing the contour plots, it was determined that the highest enzyme activity occurred at a pH of 7.5 and a temperature of 55°C. Within the specified assay range, an initial increase in pH and temperature corresponded to an increase in enzyme activity. These visual representations provide valuable insights into the effects of these variables on enzyme activity. Enzymatic activity is influenced by pH due to its impact on catalytic residues' protonation/deprotonation and structural stability
| [29] | Luisi, D. L. and D. P. Raleigh, pH-dependent interactions and the stability and folding kinetics of the N-terminal domain of L9. Electrostatic interactions are only weakly formed in the transition state for folding. Journal of molecular biology, 2000. 299(4): p. 1091-1100. https://doi.org/10.1006/jmbi.2000.3752 |
| [30] | Bjørk, A., et al., Electrostatic interactions across the dimer–dimer interface contribute to the pH-dependent stability of a tetrameric malate dehydrogenase. FEBS letters, 2003. 553(3): p. 423-426. https://doi.org/10.1016/S0014-5793(03)01076-7 |
[29, 30]
.
Figure 4. Contour plot and 3D Plot showing the interactive effect of pH and Temperature on the Enzyme activity by Papaya.
3.6.2. Effects of pH and Reaction Time
The contour plots 5a and 5b further demonstrate a strong interaction between reaction time concentrations and pH, echoing the earlier observations. The elliptical curves in
Figure 5A and 5B indicate that enzyme activity is highest at a pH of 7.5 and a reaction time of 38.5 minutes. Within the specified assay range, an increase in pH and reaction time led to a corresponding increase in enzyme activity. However, beyond the elliptical curve, the variables collectively inhibited enzyme activity due to protein denaturation. These results provide further insights into the impact of these factors on enzyme activity and highlight the importance of maintaining optimal conditions to prevent enzyme inhibition.
Figure 5. Contour plot and 3D Plot showing the interactive effect of pH and Reaction time on the Enzyme activity by papain.
3.6.3. Effects of Temperature and Reaction Time
Enzymatic activity is affected by temperature, as it influences protein stability and reaction kinetics
. The variables exhibited a significant influence on enzyme activity, as demonstrated by their respective P-values of 0.0029 and 0.0001. The peak observed in the contour diagram (
Figure 6A) indicates that the variables are within their optimal range. Interestingly, the interactive effect coefficient (AC) was found to be negative, suggesting that these variables exhibit inhibitory effects when they exceed their optimal limits. This information provides valuable insights for optimizing enzyme activity and avoiding potential inhibitory effects by carefully controlling the variables within the optimal range.
Figure 6. Contour plot and 3D Plot showing the interactive effect of Temperature and Reaction time on the Enzyme activity by Papain.
The correlation between experimental results and predicted responses is effectively demonstrated in
Figure 7A, where all predicted responses align closely with the experimental outcomes. This close correspondence suggests that the quadratic model developed in this study successfully captures the experimental results related to enzyme activity and serves as an adequate representation of the response variables under investigation.
By examining the natural logarithm (ln) of the residual sum of squares (SS) against lambda (λ), it is evident that there is a sudden dip with a minimum value at approximately 0.15 (
Figure 7B). This observation indicates that the data do not require transformation, as the current confidence interval value containing lambda (λ) is in close proximity to the optimum value. This analysis supports the conclusion that the model accurately represents the data without the need for additional transformations.
Figure 7. Relation between experimental and predicted values calculated by response surface methodological model based on Box–Behnken experimental design.
3.7. Physico-Chemical Analysis After the Dehairing Assay
Monitoring the biochemical oxygen demand (BOD) and chemical oxygen demand (COD) is crucial in evaluating the effectiveness of enzyme dehairing for pollution control, as seen in
Table 7. The enzymatic dehairing effluent contains hair and epidermal material, while the chemical effluent includes lime, sulfide, epidermal material, and degraded hair pulp. Compared to chemical-based dehairing, the BOD and COD values of the enzymatic process effluent were approximately 50% and 60% lower, respectively. This indicates that enzyme-based dehairing is a more environmentally friendly alternative to traditional chemical methods in terms of reducing pollution.
Table 7. Physicochemical analysis of effluent after treatment.
Parameter | Sheep hide |
Enzymatic treatment (Papaya) | Chemical treatment (NaS) |
BOD (ppm) | 150 ± 5.77350 | 275 ± 2.88675 |
COD (ppm) | 340 ± 5.77350 | 450 ± 0.00 |
Monitoring of BOD and COD was used to assess the enzymatic dehairing method' ability to reduce pollution and environmental contamination. The effluent that was gathered following the enzymatic dehairing cycle included very little hair and epidermal debris, whereas lime, sulfide, epidermal material, and pulp from destroyed hair were found in the chemical effluent. A study at the University of Sfax in Tunisia also demonstrated that BOD and COD improved after chemical dehairing, whereas enzymatically dehaired byproducts had limited levels of contaminants
| [26] | Ben Elhoul, M., et al., Heterologous expression and purification of keratinase from Actinomadura viridilutea DZ50: feather biodegradation and animal hide dehairing bioprocesses. Environmental Science and Pollution Research, 2021. 28: p. 9921-9934. https://doi.org/10.1007/s11356-020-11371-1 |
[26]
. An analysis of dehairing effluent from enzymatic dehairing without lime and sulfide showed that not only BOD and COD were reduced, but also sulfide toxicity was eliminated. According to the study, the effluent from the goat hide experiment was almost neutral, indicating that it was not harmful and resulted in a significant reduction of pollution as well as toxicity. This could be crucial in developing environmentally friendly tanning methods for the leather sector.
3.8. Physical Characteristics of Dyed Crusts
An examination of the physical characteristics of goat crust sections dehaired using crude enzymes and chemicals revealed notable differences between the two methods (
Table 8). The data suggests that leather treated with the enzymatic approach exhibited superior physical properties when compared to chemically treated leather. Specifically, the tensile strength, tear strength, shrinkage temperature, and percentile elongation were found to be higher in the enzymatically treated samples. These findings indicate that the use of enzymes for dehairing in leather processing can result in improved physical properties of the final product.
Table 8. Physical characteristics of dyed crusts produced from sheep hide.
Property | Method |
Enzymatic treatment | Chemical treatment |
Tensile strength (N/mm2) | 22 ± 0.3 | 15 ± 0.09 |
Percentage Elongation | 56.3 % | 48.4 % |
Tear strength (N/mm1) | 29 ±0.003 | 24 ±0.4 |
Shrinkage Temperature (°C) | 80°C | 75°C |
Regarding the physical testing for coloured crust leather, the bioprocessed leather outperformed the chemically treated leather in all categories, including tensile strength, tear strength, percentage elongation, and shrinkage temperature. Similar to this, Mouna and her colleague show that leather that had been bioprocessed using
Actinomadura viridilutea DZ50 keratinase (KERDZ) was far superior to leather that had been chemically treated. It is possible to hypothesize that leather treated with enzymes had a higher fiber opening, which improved the smoothness of dehaired skins and resulted in a significant opening up of fiber bundles
| [26] | Ben Elhoul, M., et al., Heterologous expression and purification of keratinase from Actinomadura viridilutea DZ50: feather biodegradation and animal hide dehairing bioprocesses. Environmental Science and Pollution Research, 2021. 28: p. 9921-9934. https://doi.org/10.1007/s11356-020-11371-1 |
[26]
.
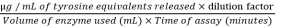 (1)
(1) 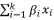 +
+ 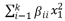 +
+ 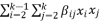 + ε(2)
+ ε(2) 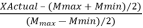 (3)
(3)