Castor seed oil is well known for its diverse medicinal and industrial uses. It is widely utilized as an additive in foods, medicine, personal care goods, lubricant and biodiesel. Nonetheless, the oil content and physicochemical properties of castor seeds depend on their genotypic varieties and geographical location. Fortunately, Ethiopia is endowed with varieties of castor seeds. However, there is a limited research on the total oil content and quality of castor seeds oil. Thus, the aim of this study was to examine the total oil content, the physicochemical characteristics and fatty acid composition of castor seeds oil grown in Jabi Tehinan Woreda, Ethiopia. The three most populous genotype castor beans were collected and subjected to soxhlet extraction using hexane solvent. The outcome showed that their genotypes determine both the amount and quality of the oil extracted. Genotype 01 (GT-01) has exceptionally the highest oil content (69.8%) while the Genotype 02 (GT-02) (47.305) and Genotype 03 (GT-03) (43.21%) have high oil contents. GT-01 has the highest (87.49%), GT-02 the second (85.17) and GT-01 (84.01) the third ricinoleic acid component. This high ricinoleic acid composition is reflected on their chemical and physical properties which are in the range of ASTM standards, making them valuable for various industrial applications.
| Published in | European Journal of Biophysics (Volume 12, Issue 1) |
| DOI | 10.11648/j.ejb.20241201.13 |
| Page(s) | 15-20 |
| Creative Commons |
This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited. |
| Copyright |
Copyright © The Author(s), 2024. Published by Science Publishing Group |
Castor Seed Oil, Fatty Acid Composition, Ricinoleic Acid, Physico-chemical Analysis
2.1. Collection of the Sample and Pre-production Process
2.2. Oil Extraction Process
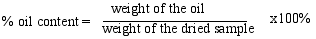
2.3. Chemical Analysis
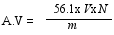
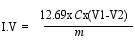
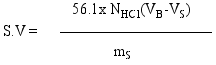
2.4. Physical Analysis
3.1. Total Oil Content
Castor bean type | Mass of the dried Castor bean sample | Mass of the extracted oil | Oil percentage |
|---|---|---|---|
GT-01 | 100g | 60.98 | 69.8% |
GT-02 | 100g | 40.73 | 47.30% |
GT-03 | 100g | 40.32 | 43.21% |
3.2. Fatty acid Content and Physicochemical Properties
Sample | Physical properties | Chemical properties | ||||||
|---|---|---|---|---|---|---|---|---|
PH | RI | SG | RV | AV | IV | SV | PV | |
GT-01 | 6.21±0.02 | 1.473±0.01 | 0.960±0.005 | 9.24±0.04 | 3.56±0.03 | 87.88±1.44 | 176±5.10 | 10.21±0.24 |
GT-02 | 6.03±0.04 | 1.470±0.03 | 0.959±0.001 | 9.55±0.03 | 3.05±0.06 | 86.00±2.65 | 181±5.33 | 10.56±0.45 |
GT-03 | 5.90±0.02 | 1.478±0.01 | 0.967±0.003 | 9.58±0.07 | 3.11±0.04 | 85.7±1.23 | 179±3.34 | 11.01±0.11 |
| [1] | Khafagi I. (2007). Variation of callus induction and active metabolite accumulation in callus cultures of two varieties of (Ricinus communis L). Biotechnol. 6: 193-201. |
| [2] | Christopher B. (1996) “The Royal Horticultural Society A-Z Encyclopedia of Garden Plants,” Dorling Kindersley, London, 884-885. A-Z encyclopedia of garden plants: Free Download, Borrow, and Streaming : Internet Archive. |
| [3] | Salihu B., Gana A., Amosun A., Shaahu S., Agboire, B. Apuyor & Oliseh A. (2023). Morphological Characterization of Castor (Ricinus ommunis L.) Accessions. Proc. Agricultural Society of Nigeria. 308-316. |
| [4] | Cosmetic Ingredient Review Expert Panel (2007). Final Report on the Safety Assessment of Ricinus communis (Castor) seed Oil. International Journal of Toxicol. 26 (suppl. 3): 31-77. |
| [5] | Weiss E. A. (2000) Castor. In “Oilseed Crops, 2nd Edition”. Blackwell Scientific Ltd, Oxford: 13-52. |
| [6] | Jeong, G. & Park, D. (2009). Optimization of Biodiesel Production from Castor Oil Using Response Surface Methodology. Applied Biochemistry and Biotechnology. 156: 431–441. |
| [7] | Ogunniyi D. (2006). Castor oil: a vital industrial raw material. Bioresources and Technology. 97(9): 1086-1091. |
| [8] | Allan G., Williams A., Rabinowicz, P., Chan, A., Ravel J. & Keim, P. (2008). Worldwide genotyping of castor bean germplasm (Ricinus communis L.) using AFLP and SSRs. Genetics Resources and Crop Evolution. 55: 365-378. |
| [9] | Papazoglou E., Alexopoulou E., Papadopoulos G. & Economou-Antonaka G. (2020). Tolerance to drought and water stress resistance mechanism of castor bean. Agronomy. 10:1580. |
| [10] | Ramos, L., Tango, J., Savi A. & Leal N. (1984). Variability for Oil and Fatty Acid Composition in Castor Bean Varieties. J. Am. Oil Chem. Soc. 61: 1841-1843. |
| [11] | Yadav P. & Anjani K (2017). Assessment of variation in castor genetic resources for oil characteristics. Journal of the American Oil Chemists' Society. 94:611-7. |
| [12] | Athumani O., Quintino A. Mgani E. & Mubofu B. (5015). Fatty Acid Profile and Physico-Chemical Parameters of Castor Oils in Tanzania. Green and Sustainable Chemistry, 5: 154-163. |
| [13] | Yusuf A., Mamza A., Ahmed A. & Agunwa U. (2015). Extraction and characterization of castor seed oil from wild ricinus communis linn. International Journal of Science, Environment and Technology. 4:1392-1404. |
| [14] | Adam O., Khalid A., Abdi A., Yaagoub I., Babiker A. & Nour A. (2020). Proximate composition, physicochemical properties and antioxidant activity of flaxseed. Annual Research & Review in Biology. 34: 1-10. |
| [15] | Kyari M. (2008). Extraction and Characterization of Seed Oils. International Agrophysics, 22: 139-142. |
| [16] | Akpan U., Jimoh A., & Mohammed D. (2006). Extraction, Characterization and Modification of Castor Seed Oil. Leonardo Journal of Sciences. 8: 43-5. |
| [17] | Orijajogun J., & Ayegba C. (2017). Physicochemical Properties and Fatty Acid Composition of Castor Bean Ricinus communis L. Seed Oil. American Journal of Applied and Industrial Chemistry. 3(1): 1-4. |
| [18] | Yeboah A, Ying S, Lu J, Xie Y, Amoanimaa-Dede H, Boateng KGA, et al (2020). Castor oil (Ricinus communis): a review on the chemical composition and physicochemical properties. Food Science and Technology. 41: 399-413. |
| [19] |
Jumat S. & Dina A. (2017). Fatty Acid Composition and Physicochemical Properties of Malaysian Castor Bean Ricinus communis L. Seed Oil. Sains Malaysiana. 39: 761–764.
http://www.ukm.my/jsm/english_journals/vol39num5_2010/contentsVol39num5_2010.html |
| [20] | Kochhar S. (1998). Economic Botany in the Tropics, 2nd ed., Macmillan India Ltd., pp. 171. 313191 (wur.nl). |
| [21] | Warra A. (2015). Physico-chemical and GC/MS Analysis of Wild Castor (Ricinus communis L.) Seed Oil. Applied Science Reports. 9(3): 123-128. |
| [22] | McKeon, T. A. (2016). Castor (Ricinus communis L.). Industrial oil crops, Elsevier. 75-112. |
APA Style
Alemayehu, Y. A., Fenta, F. W., Birhan, Y. S. (2024). Fatty Acid Composition and Physicochemical Properties of Ricinus communis Seed Oil Grown from Jabi Tehinan Woreda, Ethiopia. European Journal of Biophysics, 12(1), 15-20. https://doi.org/10.11648/j.ejb.20241201.13
ACS Style
Alemayehu, Y. A.; Fenta, F. W.; Birhan, Y. S. Fatty Acid Composition and Physicochemical Properties of Ricinus communis Seed Oil Grown from Jabi Tehinan Woreda, Ethiopia. Eur. J. Biophys. 2024, 12(1), 15-20. doi: 10.11648/j.ejb.20241201.13
AMA Style
Alemayehu YA, Fenta FW, Birhan YS. Fatty Acid Composition and Physicochemical Properties of Ricinus communis Seed Oil Grown from Jabi Tehinan Woreda, Ethiopia. Eur J Biophys. 2024;12(1):15-20. doi: 10.11648/j.ejb.20241201.13
@article{10.11648/j.ejb.20241201.13,
author = {Yihalem Abebe Alemayehu and Fekadu Wubatu Fenta and Yihenew Simegnew Birhan},
title = {Fatty Acid Composition and Physicochemical Properties of Ricinus communis Seed Oil Grown from Jabi Tehinan Woreda, Ethiopia
},
journal = {European Journal of Biophysics},
volume = {12},
number = {1},
pages = {15-20},
doi = {10.11648/j.ejb.20241201.13},
url = {https://doi.org/10.11648/j.ejb.20241201.13},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.ejb.20241201.13},
abstract = {Castor seed oil is well known for its diverse medicinal and industrial uses. It is widely utilized as an additive in foods, medicine, personal care goods, lubricant and biodiesel. Nonetheless, the oil content and physicochemical properties of castor seeds depend on their genotypic varieties and geographical location. Fortunately, Ethiopia is endowed with varieties of castor seeds. However, there is a limited research on the total oil content and quality of castor seeds oil. Thus, the aim of this study was to examine the total oil content, the physicochemical characteristics and fatty acid composition of castor seeds oil grown in Jabi Tehinan Woreda, Ethiopia. The three most populous genotype castor beans were collected and subjected to soxhlet extraction using hexane solvent. The outcome showed that their genotypes determine both the amount and quality of the oil extracted. Genotype 01 (GT-01) has exceptionally the highest oil content (69.8%) while the Genotype 02 (GT-02) (47.305) and Genotype 03 (GT-03) (43.21%) have high oil contents. GT-01 has the highest (87.49%), GT-02 the second (85.17) and GT-01 (84.01) the third ricinoleic acid component. This high ricinoleic acid composition is reflected on their chemical and physical properties which are in the range of ASTM standards, making them valuable for various industrial applications.
},
year = {2024}
}
TY - JOUR T1 - Fatty Acid Composition and Physicochemical Properties of Ricinus communis Seed Oil Grown from Jabi Tehinan Woreda, Ethiopia AU - Yihalem Abebe Alemayehu AU - Fekadu Wubatu Fenta AU - Yihenew Simegnew Birhan Y1 - 2024/04/12 PY - 2024 N1 - https://doi.org/10.11648/j.ejb.20241201.13 DO - 10.11648/j.ejb.20241201.13 T2 - European Journal of Biophysics JF - European Journal of Biophysics JO - European Journal of Biophysics SP - 15 EP - 20 PB - Science Publishing Group SN - 2329-1737 UR - https://doi.org/10.11648/j.ejb.20241201.13 AB - Castor seed oil is well known for its diverse medicinal and industrial uses. It is widely utilized as an additive in foods, medicine, personal care goods, lubricant and biodiesel. Nonetheless, the oil content and physicochemical properties of castor seeds depend on their genotypic varieties and geographical location. Fortunately, Ethiopia is endowed with varieties of castor seeds. However, there is a limited research on the total oil content and quality of castor seeds oil. Thus, the aim of this study was to examine the total oil content, the physicochemical characteristics and fatty acid composition of castor seeds oil grown in Jabi Tehinan Woreda, Ethiopia. The three most populous genotype castor beans were collected and subjected to soxhlet extraction using hexane solvent. The outcome showed that their genotypes determine both the amount and quality of the oil extracted. Genotype 01 (GT-01) has exceptionally the highest oil content (69.8%) while the Genotype 02 (GT-02) (47.305) and Genotype 03 (GT-03) (43.21%) have high oil contents. GT-01 has the highest (87.49%), GT-02 the second (85.17) and GT-01 (84.01) the third ricinoleic acid component. This high ricinoleic acid composition is reflected on their chemical and physical properties which are in the range of ASTM standards, making them valuable for various industrial applications. VL - 12 IS - 1 ER -